CCM Protein Upregulation
CCM Protein Upregulation is advancing first-in-class drug leads for age-related conditions as well as for genetically inherited diseases. Built upon highly differentiated technology platforms for protein upregulation, it is poised to become a leader in protein upregulation through oral therapeutics. Its pipeline consists of:
- Sirtuin activators: First-in-class leads that activate human sirtuin isoforms beyond SIRT1 for age-related conditions such as infertility, diabetes, and cardiovascular and neurodegenerative disorders. The company has discovered the first steady-state sirtuin activators for any substrate.
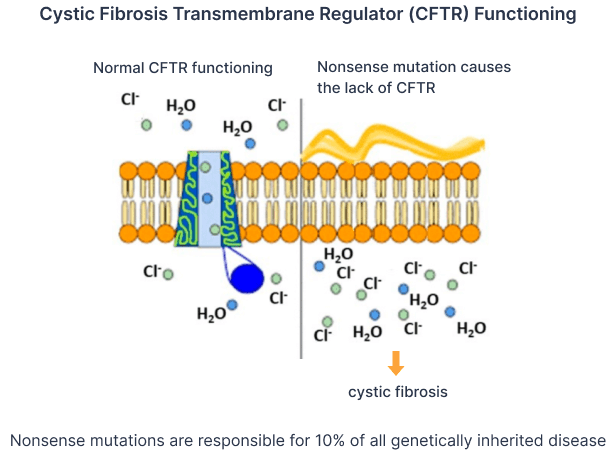
- Translational readthrough-inducing drugs: Fully lead-optimized compounds to correct nonsense mutations for Cystic Fibrosis and Duchenne Muscular Dystrophy (10% of all genetically inherited disease) that directly target genes and improves upon the toxicity and potency profiles of predecessors -- a first-in-class opportunity.
CCM-848,914,930: Translational Readthrough Inducing Drugs (TRIDs) for CFTR Nonsense Mutations
Nonsense mutations account for 10% of all genetically inherited diseases -- an estimated 30 million people worldwide. CCM Biosciences has acquired exclusive worldwide licensing rights to nonsense mutation translational readthrough drug lead compounds from the University of Palermo and the Italian Cystic Fibrosis Foundation. These drug leads represent a first-in-class opportunity for the treatment of such diseases. CCM Bio will take these leads through the development process for two orphan diseases -- Cystic Fibrosis and Duchenne’s Muscular Dystrophy
The only competitor lead for Cystic Fibrosis, Ataluren, was rejected by the FDA because of dose-limiting toxicity. Vertex Pharmaceuticals Inc’s (NASDAQ: VRTX) recently FDA Approved drug Trikafta, eligible for treating the remaining 90% of Cystic Fibrosis patients is non-competing with CCM Bio’s leads.
The consortium, comprised of the University of Palermo and the Italian Cystic Fibrosis Foundation, has emerged as a global leader in translational readthrough inducing drugs (TRIDs). This group, comprised of leading faculty members including Dr. Ivana Pibiri and Dr. Andrea Pace has applied state-of-the-art computational modeling and biophysical measurements to achieve a detailed understanding of the Modes of Action (MoA) and design of new leads. This is evidenced by their extensive publications and patents in this space spanning the last decade. Due to their significant differences with respect to conventional inhibitors, the MoA of these TRIDs has been hotly debated and proven elusive to full characterization. The research work of this consortium has elucidated several fundamental principles of this MoA, which have been exploited to design more effective patented TRIDs. These overcome the dose-limiting toxicity of precursors while preserving efficacy.
- First-in-class Compound for Nonsense Readthrough Mutations Targeting Cystic Fibrosis
- ACTIVITY OF MOLECULES CCM-848, CCM-914, and CCM-930
CCM-848,914,930 are nonsense mutation translational readthrough drug compounds for genetically inherited diseases. Acquired through exclusive licensing rights from the University of Palermo and the Italian Cystic Fibrosis Foundation.
MoA CCM’s drug lead binds directly to genes and upregulate expression of the associated proteins thus overcoming the ‘undruggable’ limitations offered by conventional small molecules that target proteins.
Key Scientific Data:
- Toxicity studies in mice and zebrafish and biodistribution studies completed.
- Non-toxic in the in vivo model.
- Demonstrated significantly lower cytotoxic effect in animal models than Ataluren which was rejected by FDA for insufficient efficacy / dose-limiting toxicity.
- Demonstrated efficient readthrough activity in cell model
- Metabolically stable.
- CCM’s medicinal chemistry partner developed novel patentable compounds to overcome dose-limiting toxicity while preserving efficacy.
Competitive Position & Commercial Opportunity:
Nonsense mutations account for 10% of all genetically inherited diseases - an estimated 30 million people worldwide. Advantages over gene therapy approaches due to simplicity of drug delivery.
Duchene Muscular Dystrophy is an alternative indication and offers the benefits of an orphan indication.
Patent: Patent granted from USPTO and EPO. Patent expiry 2039.
Next Steps & Key Milestones:
IND enabling studies for the indication of Cystic Fibrosis underway in collaboration with the Italian Cystic Fibrosis Foundation.
Partner: University of Palermo & The Italian Cystic Fibrosis Foundation
Principal Investigator: Prof. Ivana Pibiri (PhD)
Scientific Publications:
- Pibiri, I. et al. Targeting nonsense: optimization of 1,2,4-oxadiazole TRIDs to rescue CFTR expression and functionality in cystic fibrosis cell model systems. Int. J. Mol. Sci. 21: 6420, 2020.
- Pibiri, I. et al. Exploring the readthrough of nonsense mutations by non-acidic ataluren analogs selected by ligand-based virtual screening. Eur. J. Med. Chem. 122: 429-435, 2016.
Enabling Platform: Small Molecule Discovery Platform
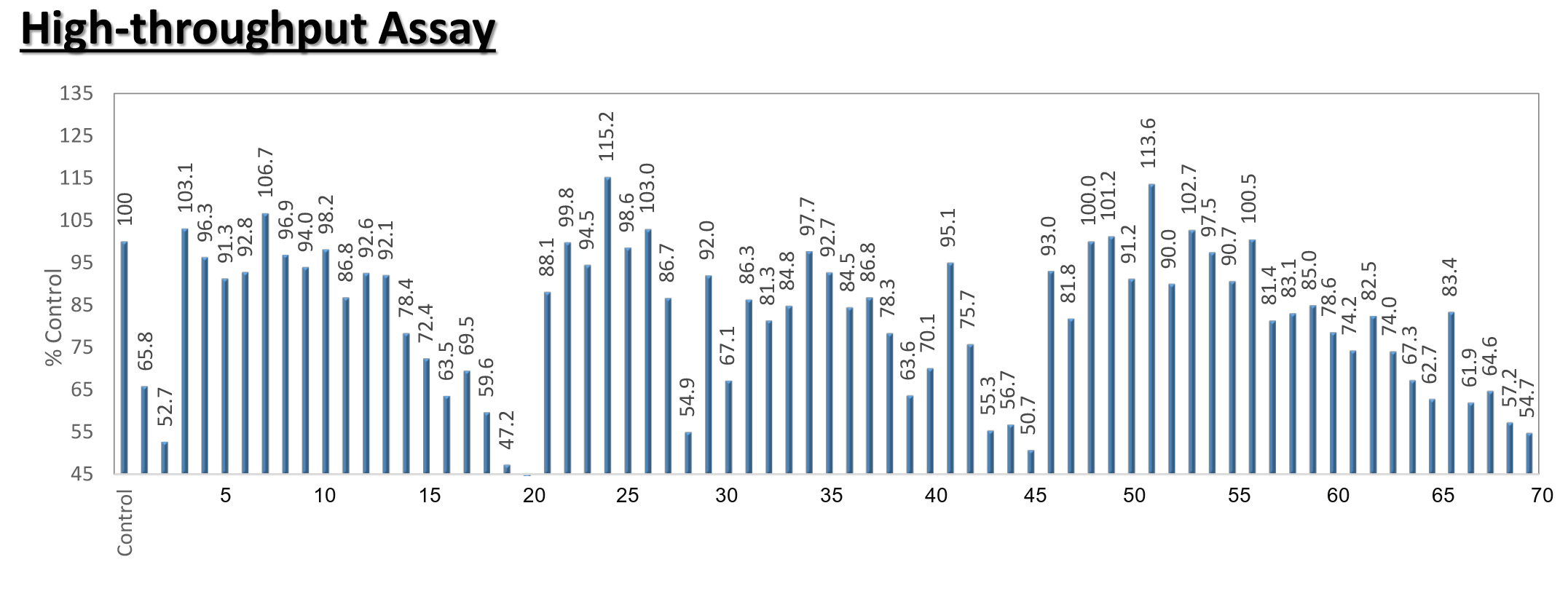
Testing the readthrough activity on the CFTR mRNA expression level by Fluc Cell based assay
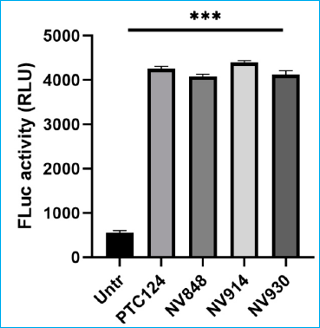 PTC124 = Ataluren
PTC124 = AtalurenNV848 = CCM-848
NV914 = CCM-914
NV930 = CCM-930
Histogram shows luciferase (FLuc) activity (RLU) after 24 h of exposition to PTC124, NV848, NV930, and NV914 (all at the concentration of 12 μM) in HeLa FLuc-opal transfected
-
PTC124: Only translational readthrough drug candidate in clinical trials
-
Rejected by FDA for insufficient efficacy / dose-limiting toxicity
-
CCM’s medicinal chemistry partners, world experts on TRIDs, developed novel patentable compounds to overcome
dose-limiting toxicity while preserving efficacy
In Vivo Toxicity in Zebrafish Model
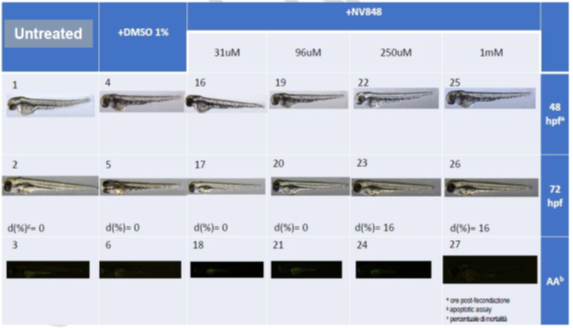
At 48 and 72 hours post-fertilization (hpf), CCM-848 exposed embryos were morphologically indistinguishable from control unperturbed and DMSO-treated larvae. By contrast, other TRID compounds tested at concentrations equal or above 12 μM caused developmental aberrations in all of the observed specimens
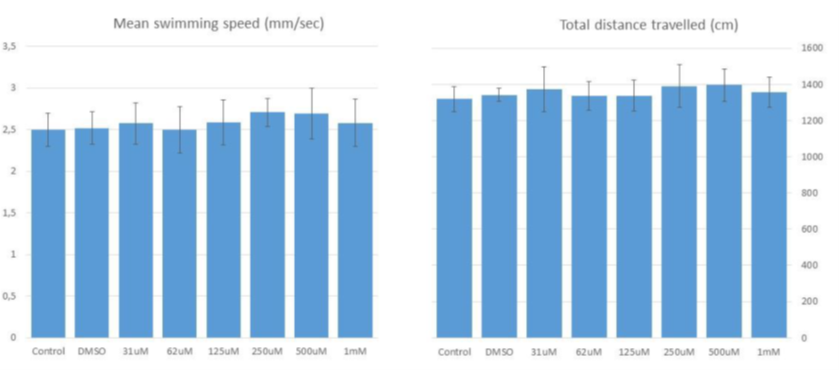
NV848 treatment at the dosage in the range of 31 μM - 1 mM did not affect the swimming velocity and total distance travelled by zebrafish larvae in the time window examined. No dose-limiting toxicity to limit therapeutic window
First-in-class nonallosteric activators of Sirtuin enzymes
Allosteric activation is currently the only means by which small molecule drugs can enhance the activity of enzymes. However, the vast majority of potential drug targets are not amenable to allosteric activation due to the lack of an allosteric site. However, it is known that the ability to upregulate a variety of such enzymatic targets could enable the treatment of a host of associated diseases. CCM Biosciences’ proprietary drug discovery platform for non-allosteric activation of enzymes focuses on the activation of the so-called sirtuin enzymes, which have been shown to play a central role in age-related diseases, such as infertility, diabetes, neurodegenerative disorders, and cardiovascular diseases. Multiple novel lead compounds have been identified which are currently undergoing optimization through preclinical studies.
CCM Bio’s proprietary drug discovery platform for enzyme activation is comprised of computational and experimental components. The computational component builds upon two publications in the Proceedings of the National Academy of Sciences (PNAS) by Dr. Raj Chakrabarti (Founder and CEO of CCM Biosciences) and Prof. Richard Friesner of Columbia University (founder of the computational drug design company, Schrodinger Inc, NASDAQ: SDGR). The computational component is further complemented with an experimental ultrahigh-throughput screening component based on DNA-Encoded Libraries (DELs). DEL libraries, which apply the principles of Darwinian selection to small molecule drug compounds, are custom-generated for several of CCM Bio’s drug discovery programs and can include billions of compounds per library. State-of-the-art machine learning algorithms are applied to this big data to guide the Darwinian evolution process towards optimal drug leads faster and more efficiently. The application of DEL in conjunction with biophysics and machine learning-based drug discovery algorithms predates that of competitors.
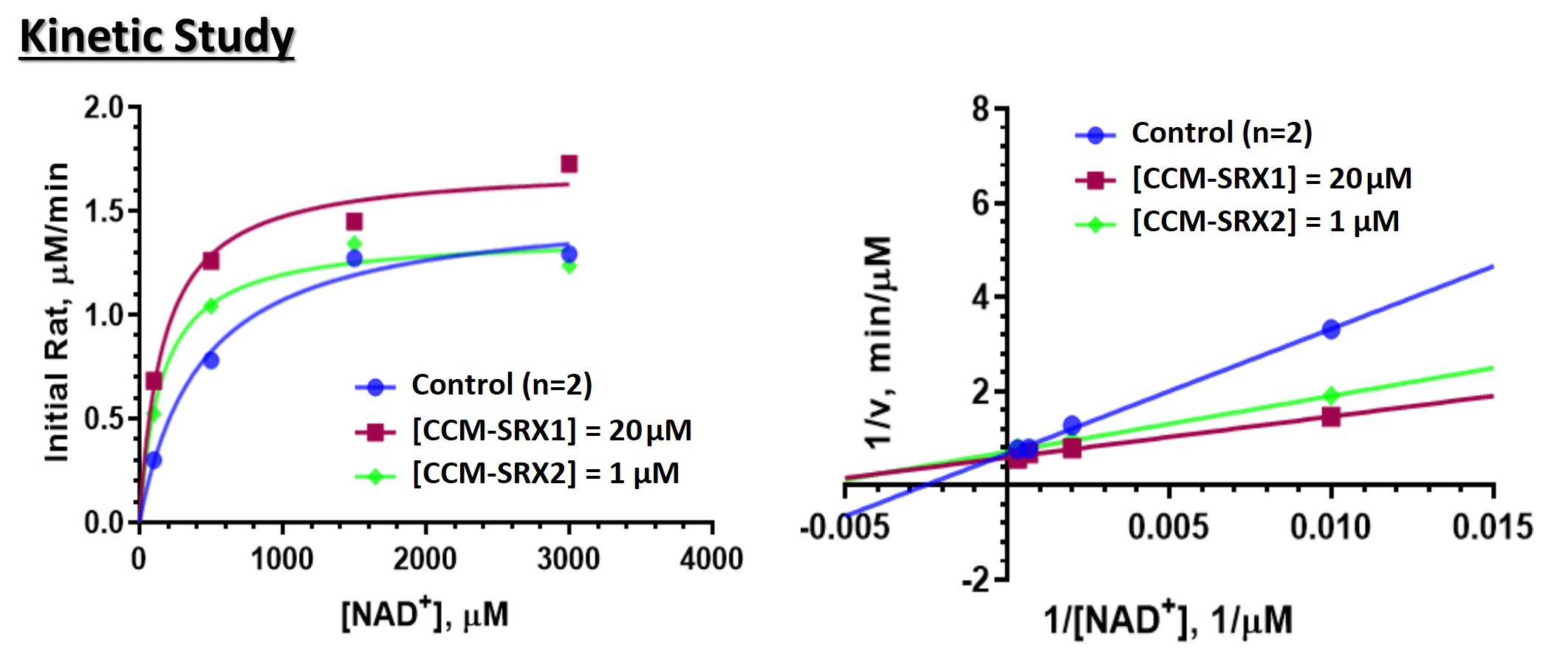
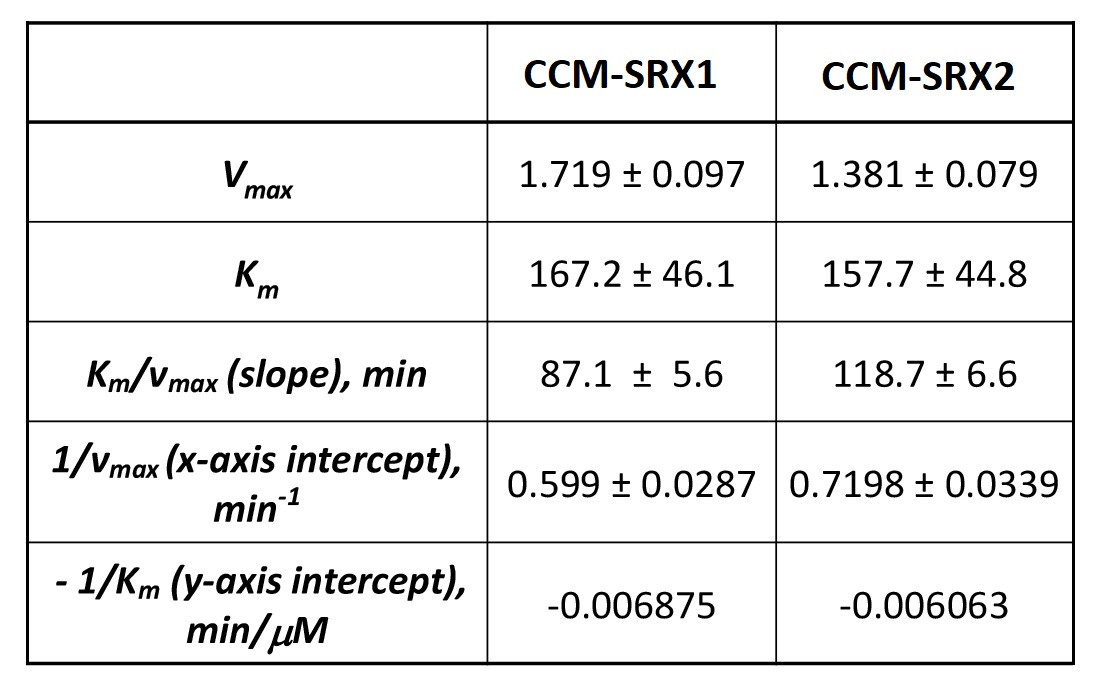
Among the 7 mammallian sirtuin enzymes SIRT1-7, only SIRT1 has an allosteric site. In the absence of nonallosteric activators, to date sirtuin activation has been pursued pharmaceutically for SIRT1 (by Sirtris Pharmaceuticals, now GSK) by allosteric activation and for SIRT3 by nutraceutical NAD supplements which are not drugs (by Elysium Health). Using the above integrated drug discovery platform, CCM Bio has discovered novel hits for activation of SIRT3 – the major mitochondrial sirtuin – that are more potent and activate under a much wider range of physiologically relevant conditions than the small number of hits that were previously identified empirically in the literature. The SIRT1 activators from Sirtris, for instance, only work on a small number of substrates. Moreover, our platform may be used to identify activators of multiple other SIRT enzymes and for much wider variety of substrates. We experimentally characterized the mode of action for such compounds, uncovering for the first time the mechanism whereby they can activate enzymes without allosteric sites. In particular, these compounds have been shown to increase the binding affinity of the essential cellular metabolite nicotinamide adenine dinucleotide (NAD) to the enzyme, thereby potentially mitigating diseases caused by the depletion of NAD with age.
- Novel Sirtuin Activating Drug Lead Targeting a Broad Range of Age-related Diseases
- Mechanism of Action of SIRT3 Activators
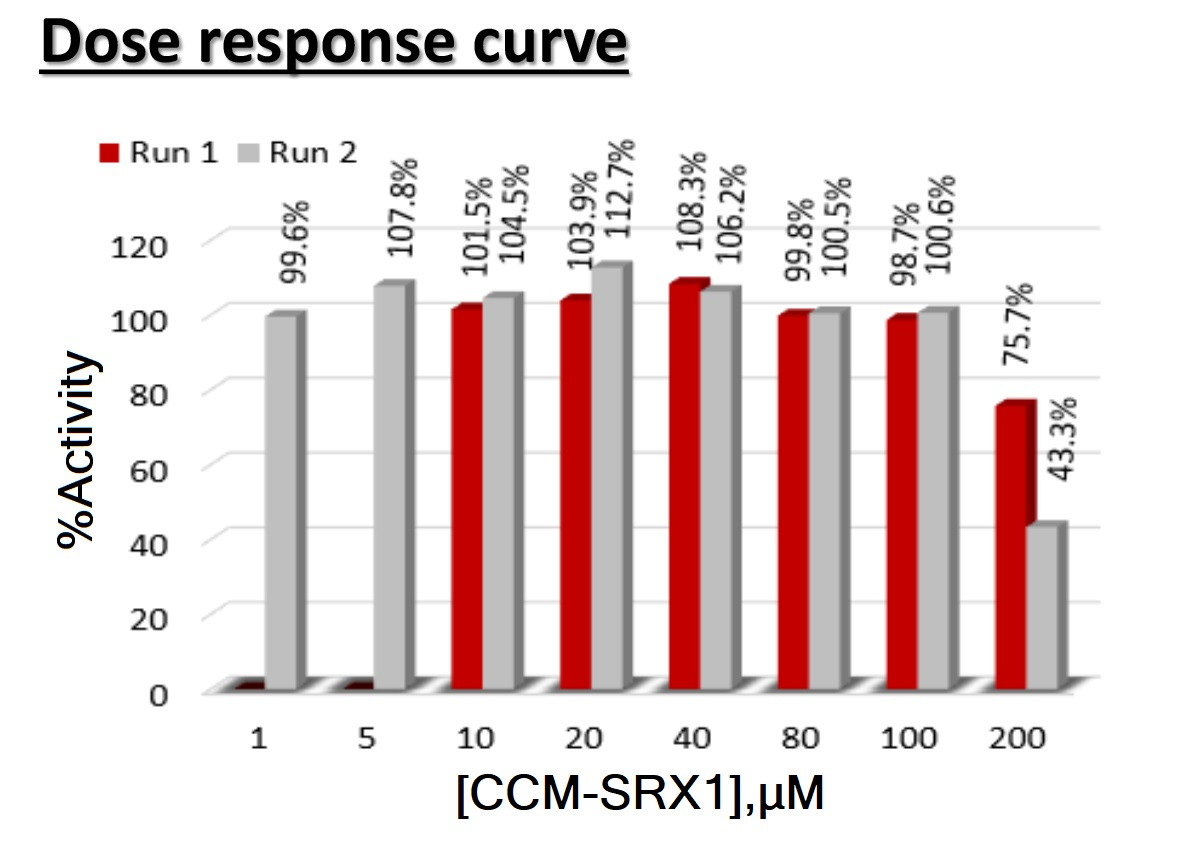
CCM-SRX is a SIRT3 activator and targeting a variety of age-related diseases such as infertility, diabetes, neurodegenerative disorders and cardiovascular diseases.
MoA Sirtuin enzymes have been shown to play a central role in age-related diseases, but most of these enzymes are considered “undruggable” due to the need to activate the enzymes in the absence of known allosteric sites. Sirtuins have been shown to increase the binding affinity of the essential cellular metabolite Nicotinamide Adenine Dinucleotide (NAD) to the enzyme, thereby potentially mitigating diseases caused by the depletion of NAD with age. Upregulation/activation of sirtuins has been shown to increase animal lifespan.
CCM’s novel drug candidates activate SIRT3, the major mitochondrial sirtuin, that are several fold more potent than the nutraceutical NAD supplements and activate under a much wider range of physiologically relevant conditions
Key Scientific Data:
- Targets activation of SIRT3
- Identified small molecule hits with 200% activation effect at 1 uM
- Targets all SIRT family members – an advantage over the competition
- Works with all substrates/disease pathways
- Higher potency relative to NAD+ supplements
- SIRT activators have been tested in older mice and shown to reverse signs of aging
- Toxicity: Large therapeutic window (for IVM/IVF)
Patent: Patents filed/granted: 63/296,271; 2020/0370089 A1; 2022/15/759,646
Competitive Position & Commercial Opportunity:
CCM’s sirtuin activating drug leads were discovered in-house using its proprietary drug discovery platform for non-allosteric activation of enzymes. No competing proprietary leads for SIRT3.
Lead with In Vitro Fertilization (IVF) and In Vitro Maturation (IVM)
and other indications include Alzheimer’s (currently in lead optimization) and Parkinsons.
The U.S. is the second largest fertility market followed by Japan, and the global market size is estimated to reach $36 billion by 2028.
Next Steps & Key Milestones:
In-vivo studies for In-Vitro Maturation (IVM) and In-Vitro Fertilization (IVF) and clinical trials for Noninvasive Preimplantation Genetic Testing (niPGT) underway.
Principal Investigator: Dr. Raj Chakrabarti (PhD)
Scientific Publications:
- Sequence optimization and designability of enzyme active sites. Raj Chakrabarti, Alexander M. Klibanov and Richard A. Friesner. Proc. Natl. Acad. Sci. USA 102 (34) 12035-12040 (2005)
- Computational prediction of native protein ligand-binding and enzyme active site sequences. Raj Chakrabarti, Alexander M. Klibanov and Richard A. Friesner. Proc. Natl. Acad. Sci. USA 102 (29) 10153-10158 (2005)
- Mechanism of inhibition of the human sirtuin enzyme SIRT3 by nicotinamide: computational and experimental studies. Xiangying Guan, Ping Lin, Eric Knoll and Raj Chakrabarti. PLOS ONE 9 (9): e107729 (2014)
- Biophysical characterization of hit compounds for mechanism-based enzyme activation. Xiangying Guan, Alok Upadhyay, Sudipto Munshi and Raj Chakrabarti. PLOS ONE 13 (3): e0194175 (2018)
- Discovery of novel compounds as potent activators of Sirt3. Célina Reverdy, Gaetan Gitton, Xiangying Guan, Indranil Adhya, Rama Krishna Dumpati, Samir Roy, Santu Chall, Anisha Ghosh, Gauthier Errasti, Thomas Delacroix and Raj Chakrabarti. Bioorganic and Medicinal Chemistry 73 116999 (2022)
- Molecular system identification for enzyme directed evolution and design. Xiangying Guan and Raj Chakrabarti. J Chem Phys 147 124106 (2017)
-
Pillai, V. B. et al. Honokiol blocks and reverses cardiac hypertrophy
in mice by activating mitochondrial SIRT3, Nat. Commun. 6: 6656-6672, 2015.
Enabling Platform: Small Molecule Discovery Platform

